Seca (Germany) - Quality management satisfies global requirements and secures seca competitive advantages.
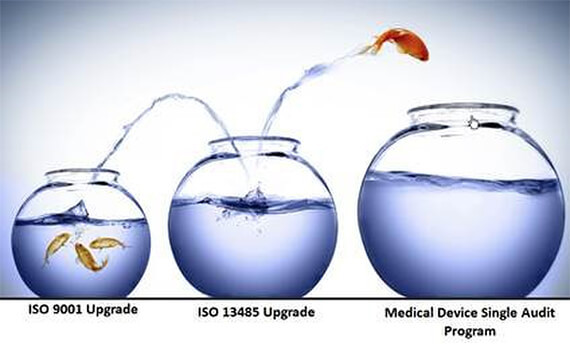
The German and French quality management systems of seca, a leading manufacturer of medical measuring systems and scales, have been certified according to the revised standards ISO 9001 and ISO 13485 in an audit conducted by TÜV-Süd. In addition, the quality management system at seca headquarters in Hamburg received the Medical Device Single Audit Program (MDSAP) certificate. It shows that seca officially satisfies not only the legal requirements in Europe, but also in the USA, Canada, Australia, Japan and Brazil.
International law makers and government agencies joined up in MDSAP in order to reduce the number of QM system audits in the different target markets. "MDSAP certification allows us to minimize the redundant efforts involved with fulfilling country-specific quality requirements as the participating national registration agencies accept the MDSAP audit report in place of their individual inspections. In future we will be able to accelerate the process of gaining market approvals," said Mark Sonnenkalb, Head of Quality Services at seca. "Meeting the toughest quality standards is part of the seca DNA. The certificates are now the official proof that our products fulfill the requirements of the MDD in Europe and of the five countries participating in MDSAP. Beyond that, the certification secures market access in those countries now and in future."
The seca portfolio includes classic medical measuring systems and scales along with innovative solutions such as networked vital signs monitors, measuring stations that communicate with each other, service and software systems that simplify everyday medical work and the medical Body Composition Analyzer (mBCA), which has the potential to revolutionize diagnostics and treatment with the use of Bioelectrical Impedance Analysis. seca medical products in Classes I, Im and IIa must fulfill strict quality requirements. The recently conducted audit has confirmed that they do.


























Interested? Submit your enquiry using the form below:
Only available for registered users. Sign In to your account or register here.